Organic Chemistry Made Ridiculously Simple
Also available on
Description
A clear, concise overview of the most important principles and reactions in Organic Chemistry. The purpose of this book is to help make the understanding of organic chemistry successful, easier, and even enjoyable! The approach assumes that organic chemistry is based on a firm foundation of simple and intuitive principles, and that new information can be incorporated, and problems can be solved, by directly applying these basic principles.
Emphasizes understanding over rote memorization and facilitates the rapid and enjoyable learning of this difficult subject. Ideal for all undergraduate college Organic Chemistry courses or as a “brush-up” to assist understanding in med school biochemistry.
Author(s)
Gene A. Davis, Ph.D.
Gene A. Davis received his doctorate in organic chemistry from the University of Chicago (1968). He worked as an industrial research chemist, senior scientist, R and D director, and consultant for 30 years, and he has authored 28 publications and patents. He has taught general, organic, and clinical chemistry, as well as biochemistry, at the University of Massachusetts (Lowell) and several other colleges and universities. His book exemplifies his unique and effective teaching approach to organic chemistry.
Details
Pages: 210
Publication: Edition 1 (November 1, 2013)
Language: English
ISBN: 9780940780422 eISBN: 9781935660149
Table of contents
PART 1. PRINCIPLES OF ATOMIC AND MOLECULAR STRUCTURE AND CHEMICAL REACTIVITY.
Chapter 1. Atomic Structure
Chapter 2. The Octet Rule and Lewis Structures
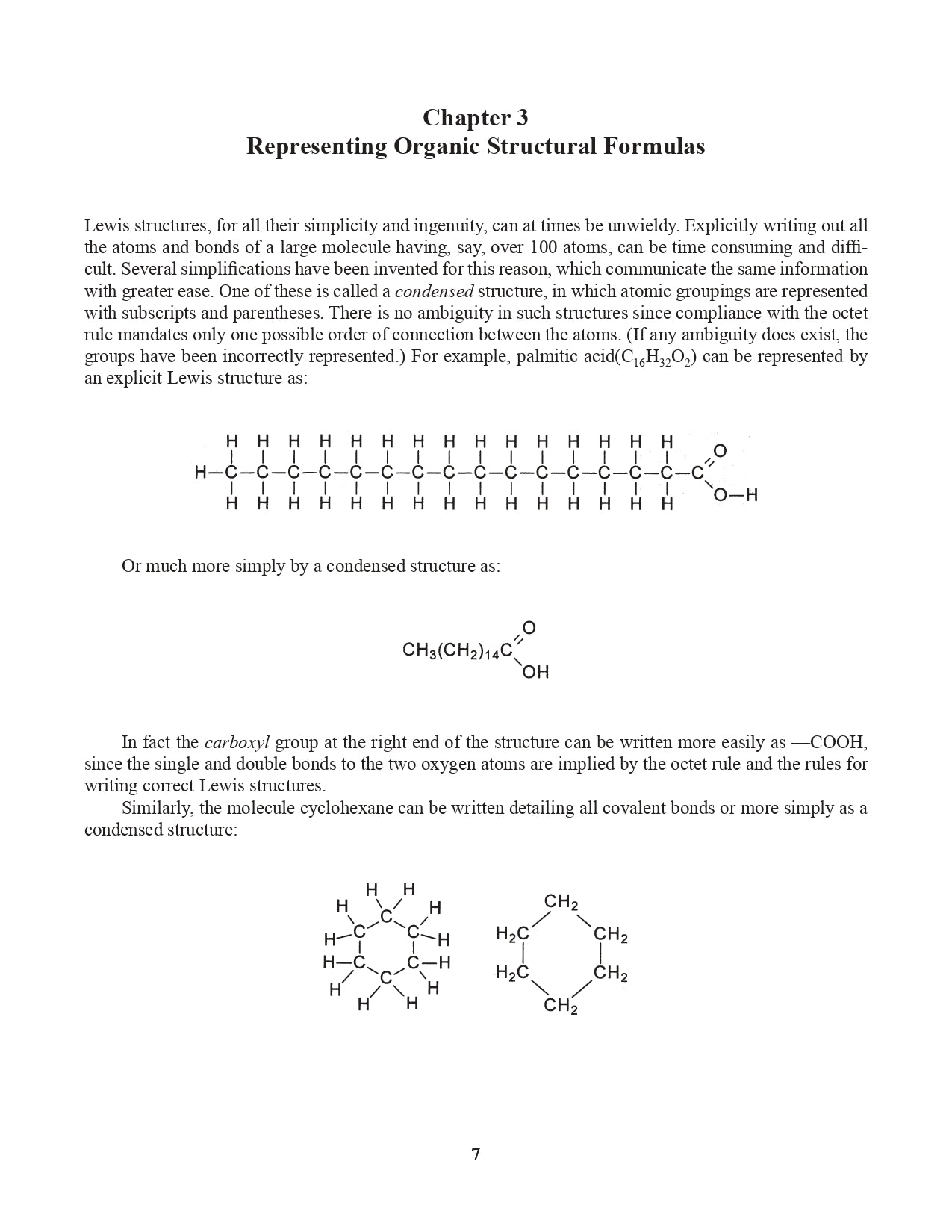
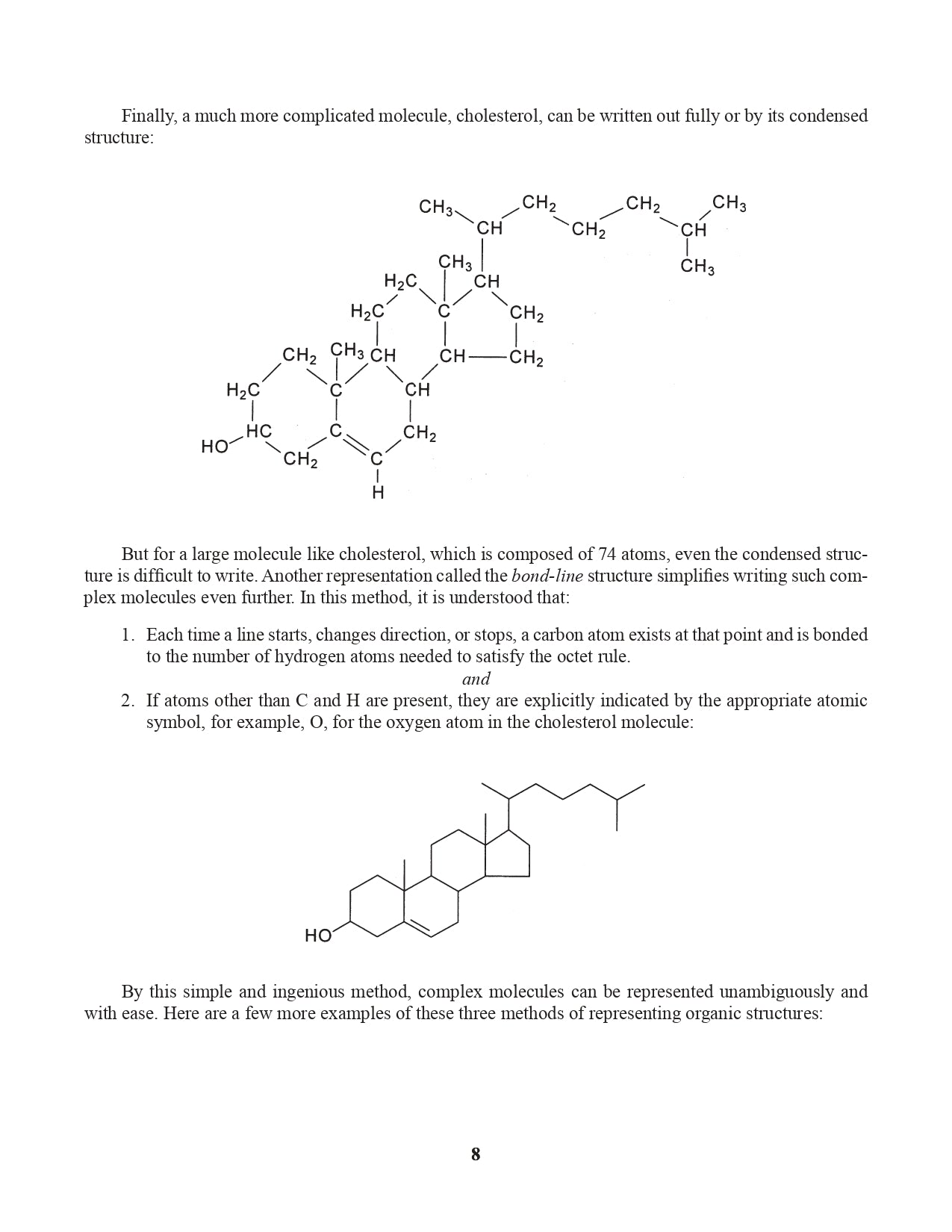
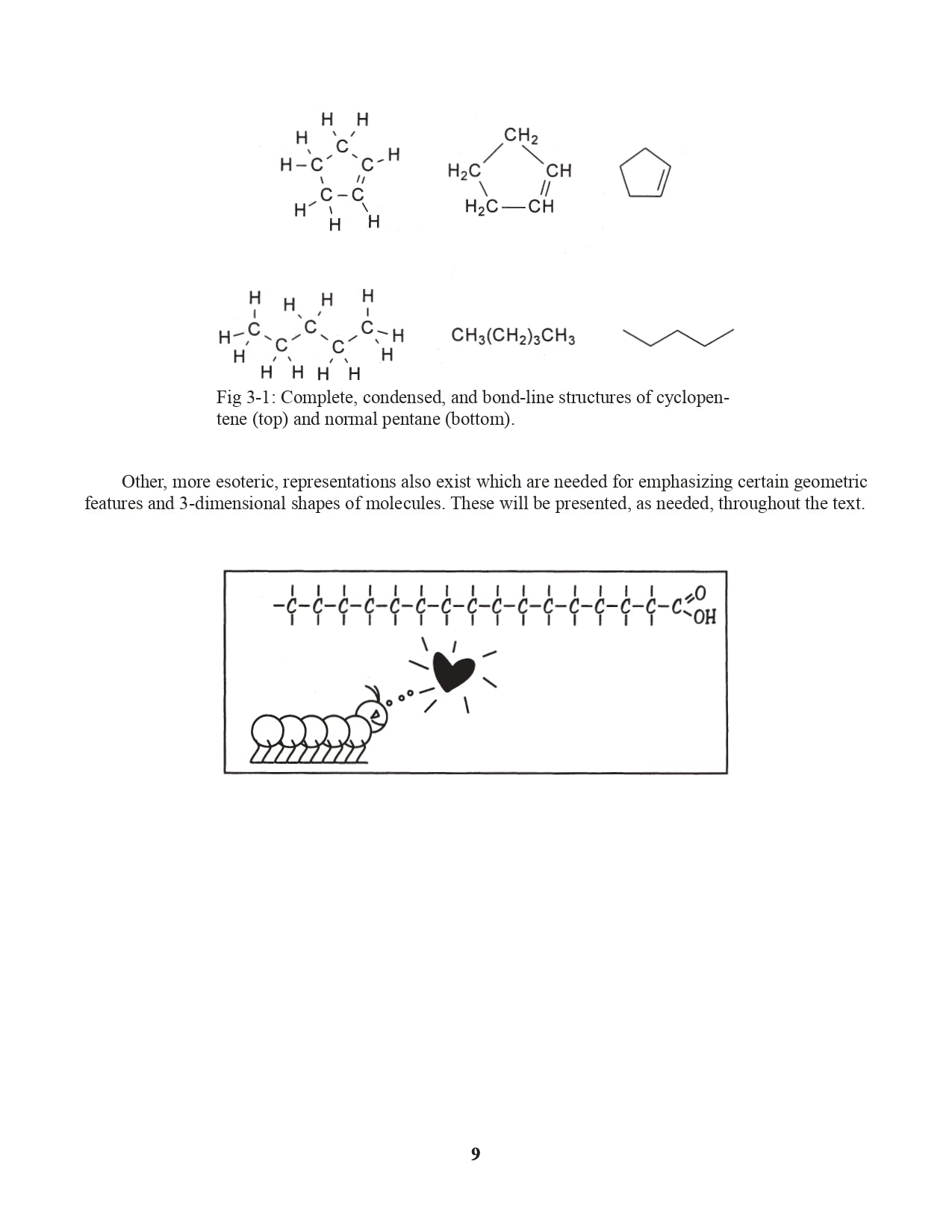
Chapter 3. Representing Organic Structural Formulas
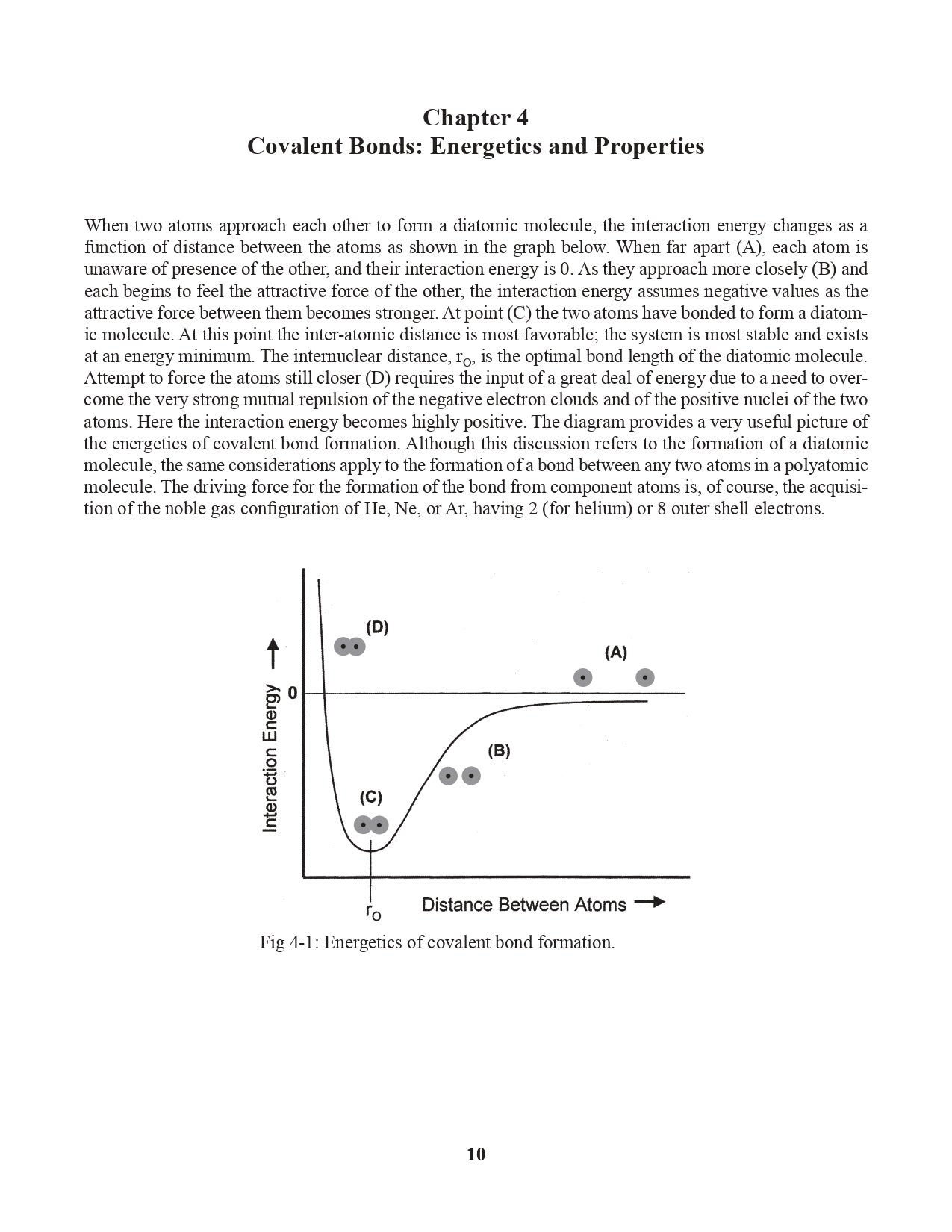
Chapter 4. Covalent Bonds: Energetics and Properties
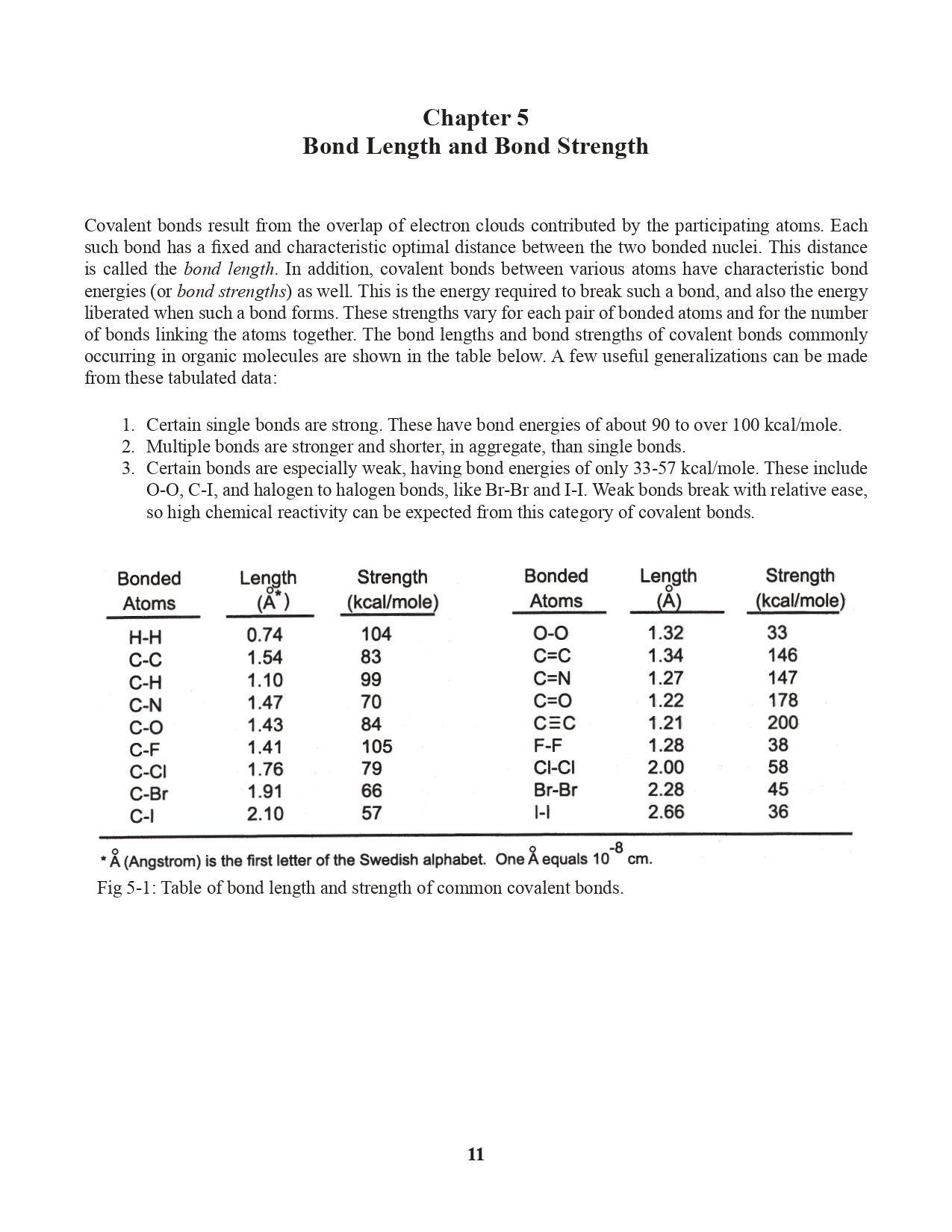
Chapter 5. Bond Length and Bond Strength
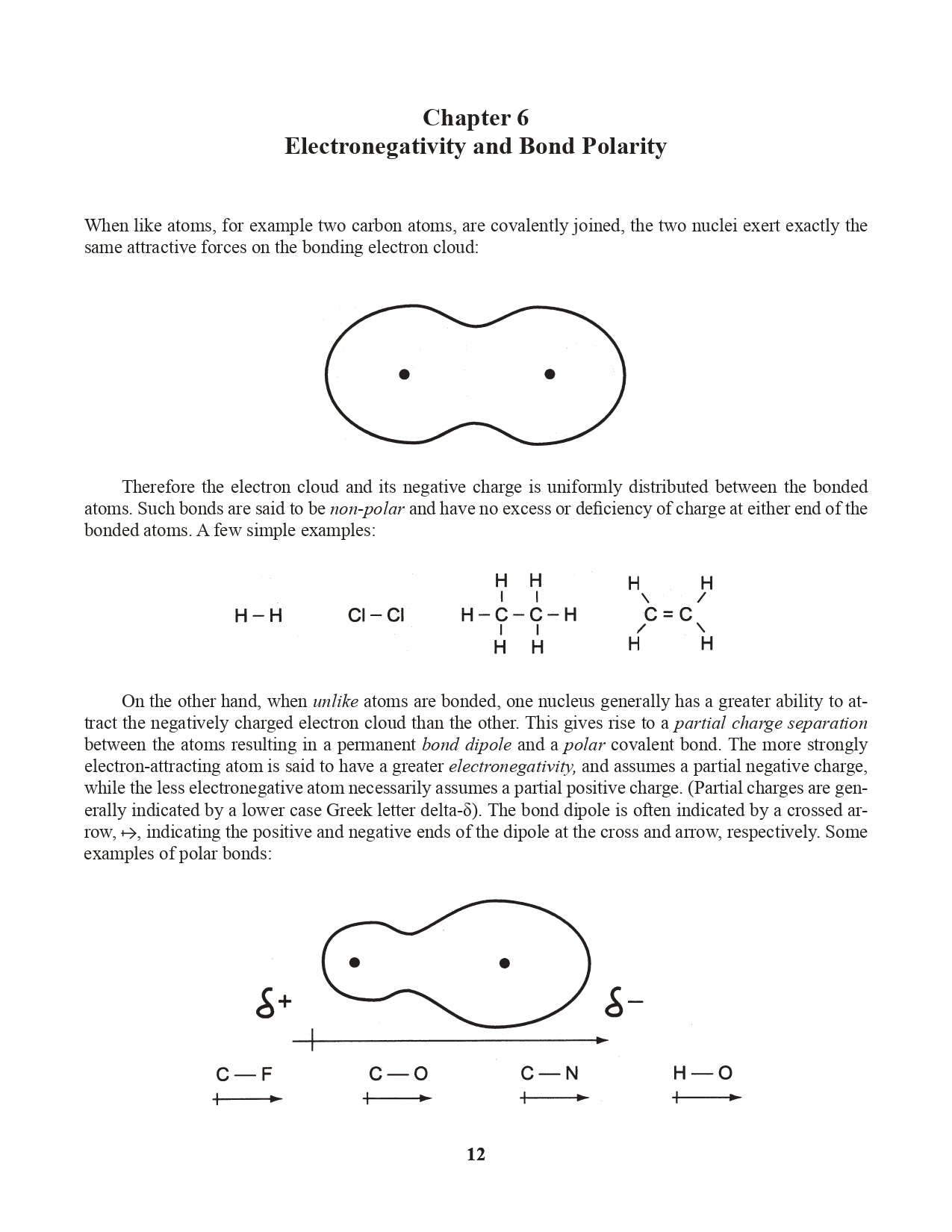
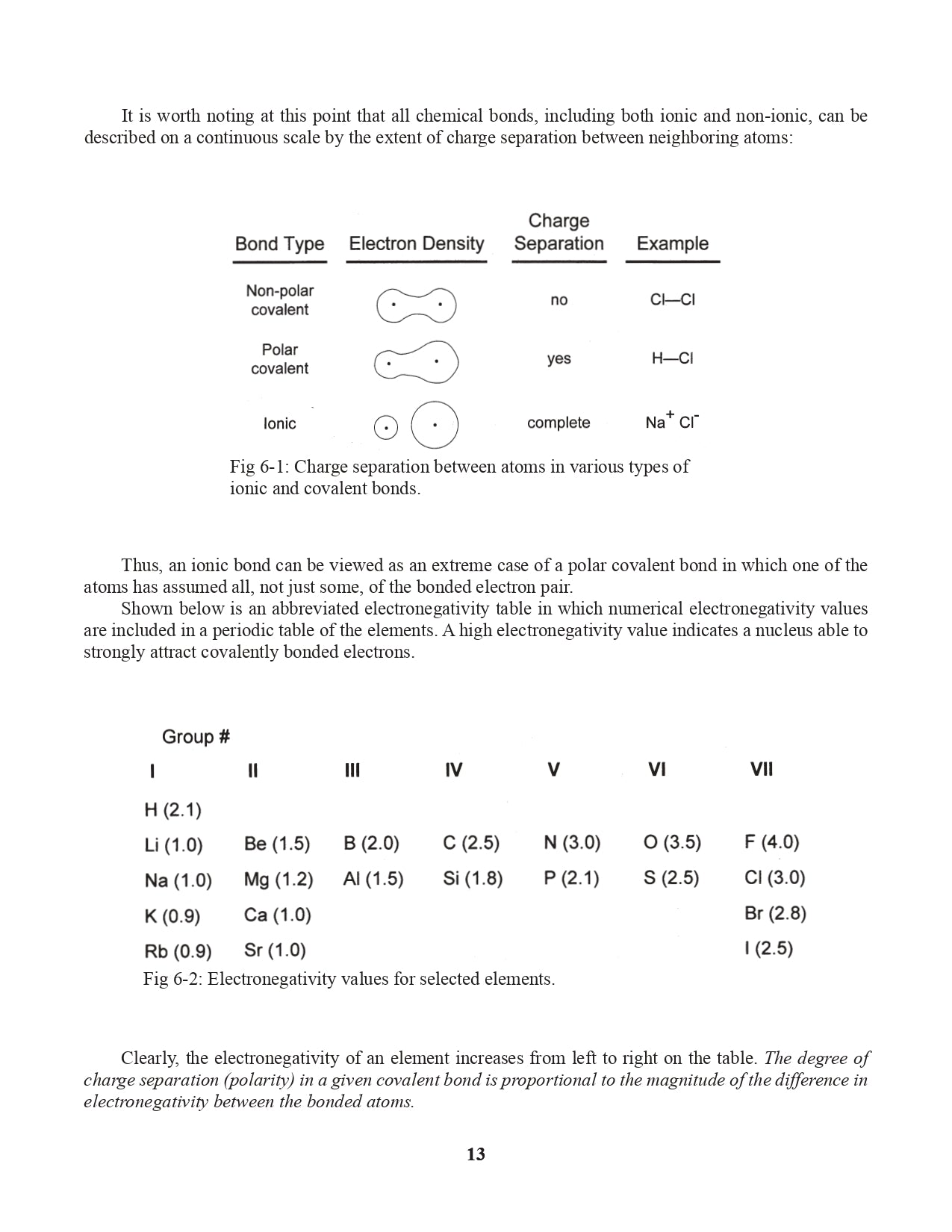
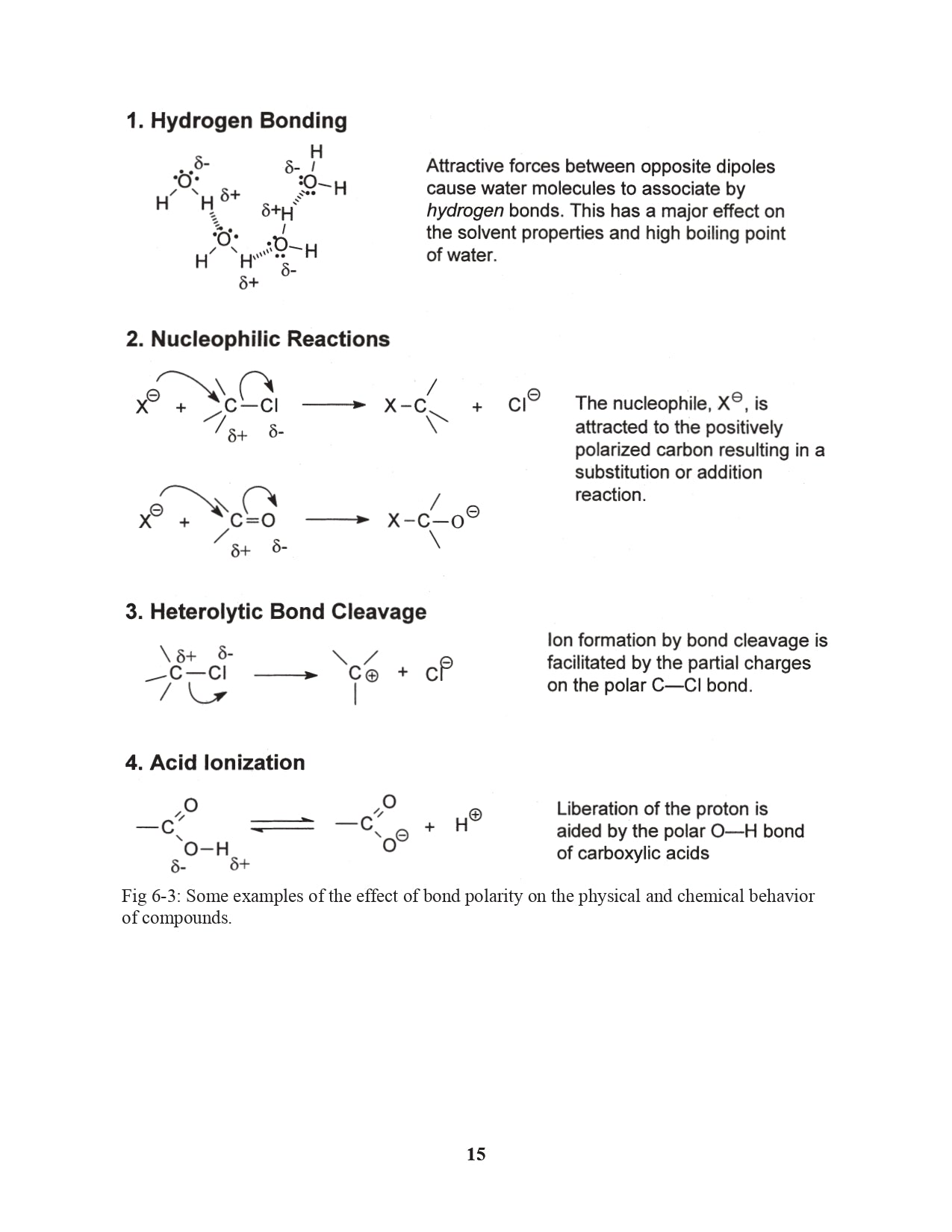
Chapter 6. Electronegativity and Bond Polarity
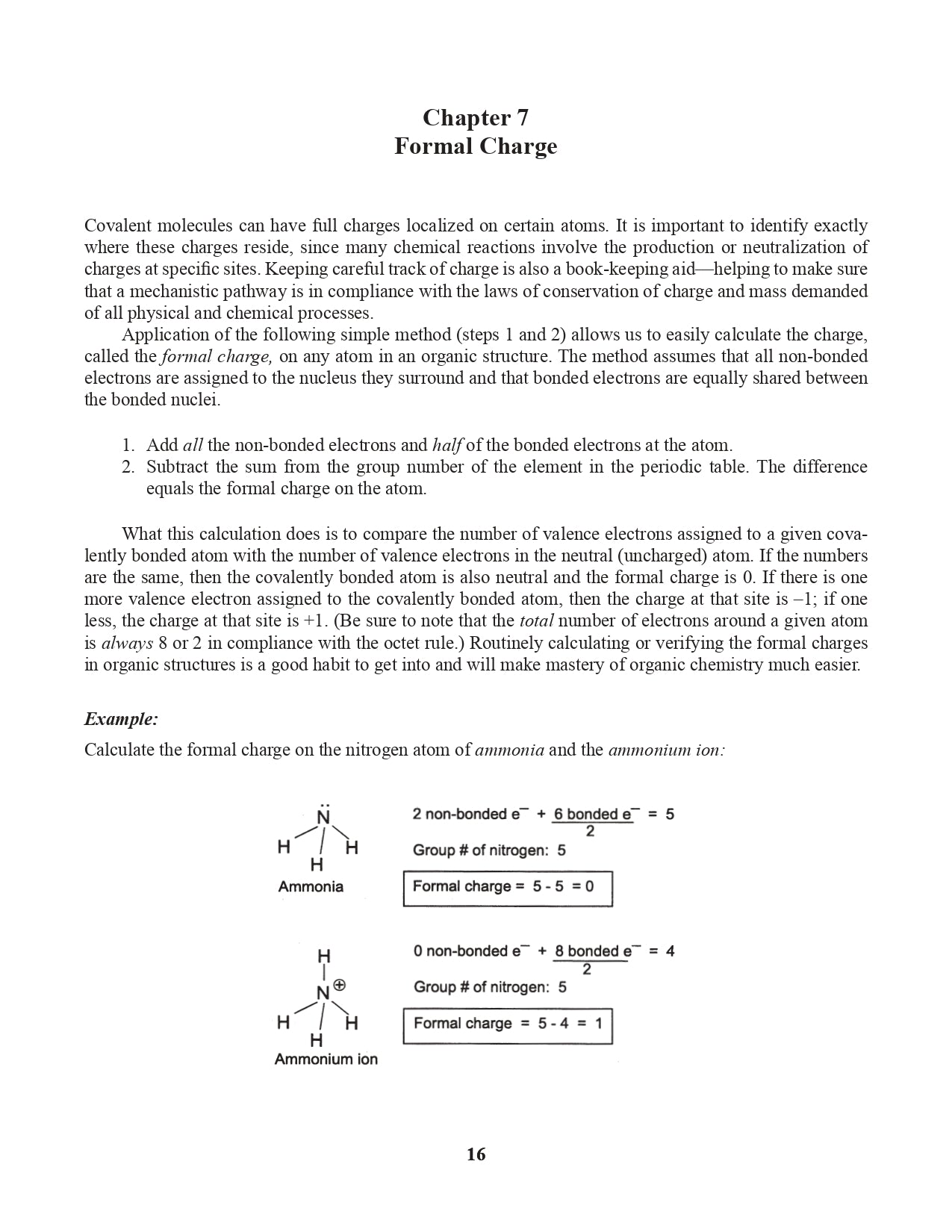
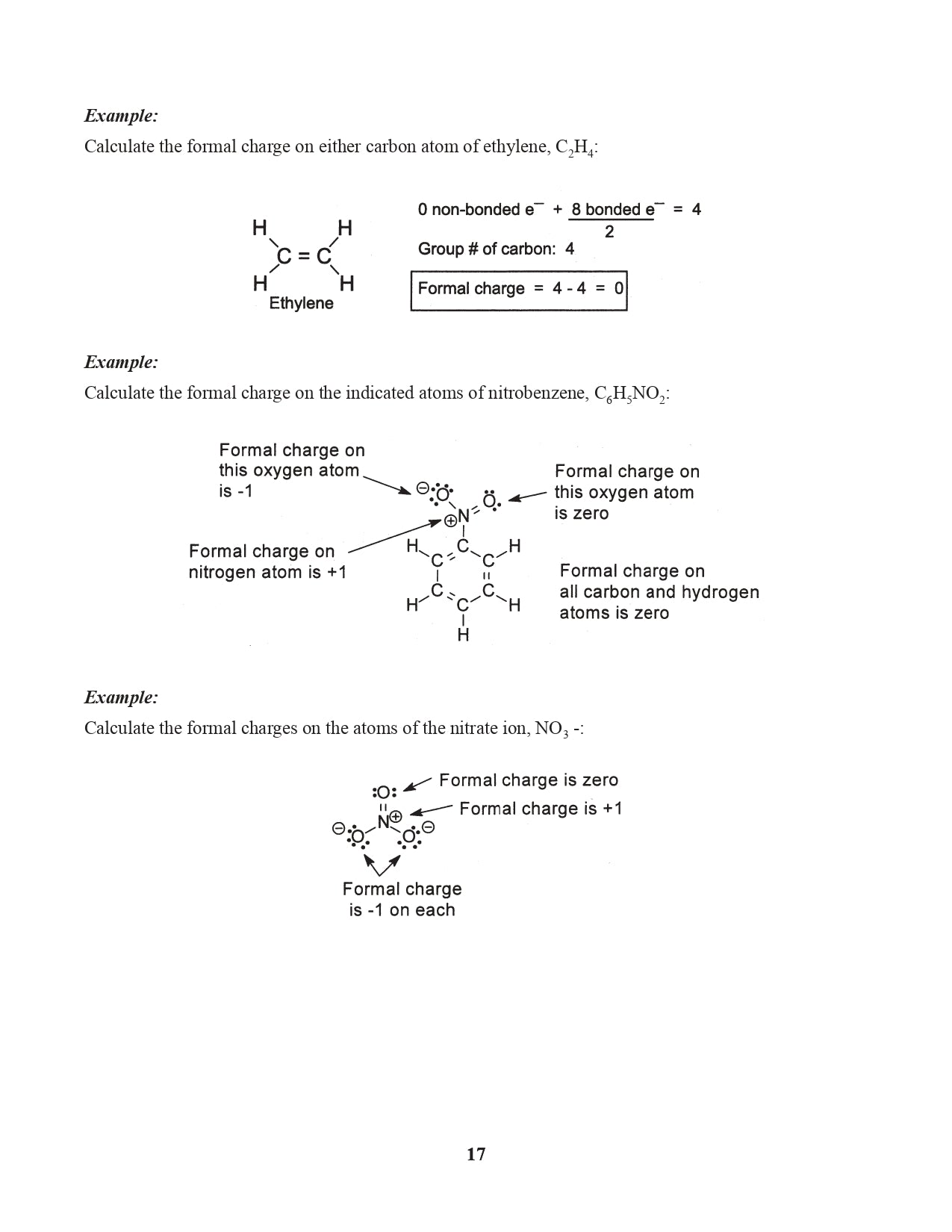
Chapter 7. Formal Charge
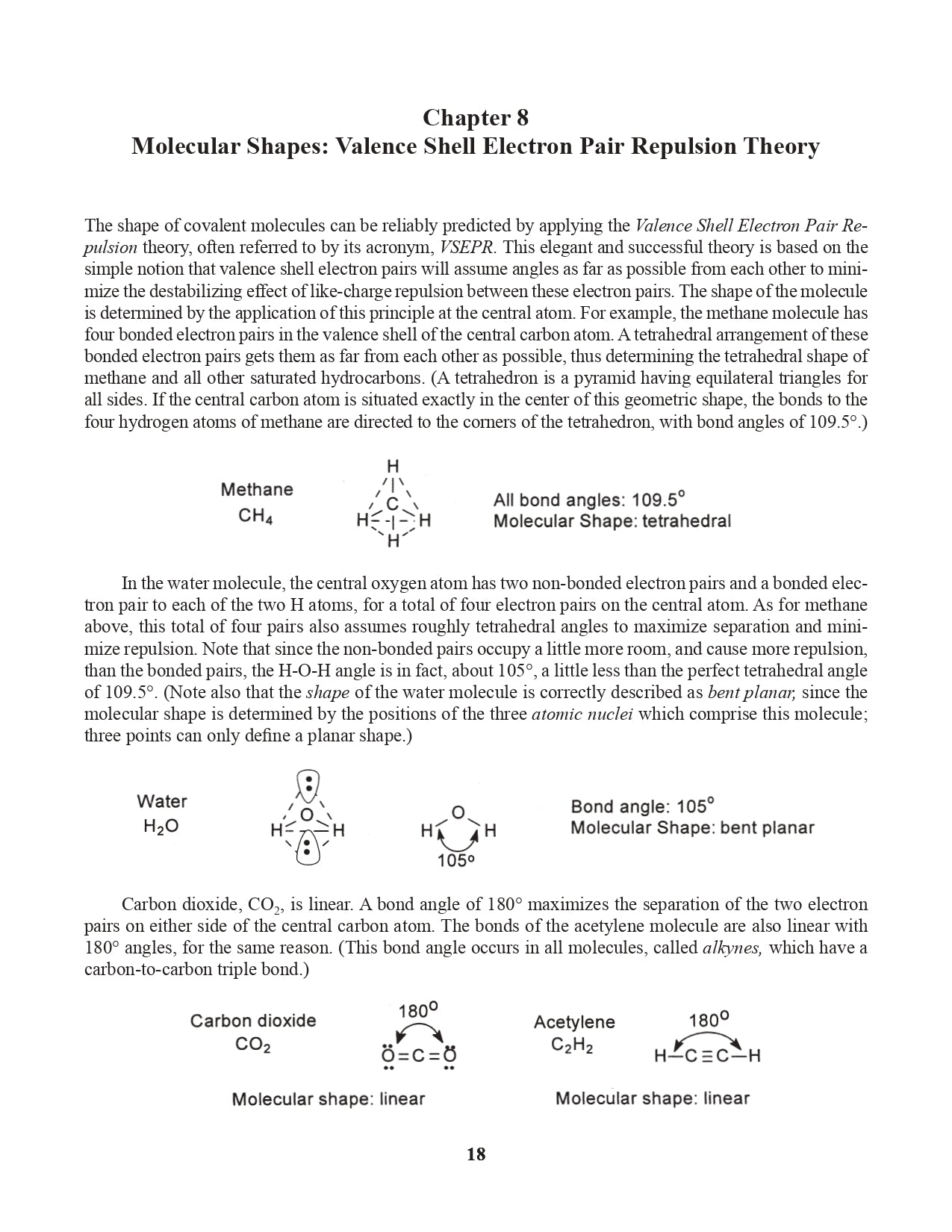
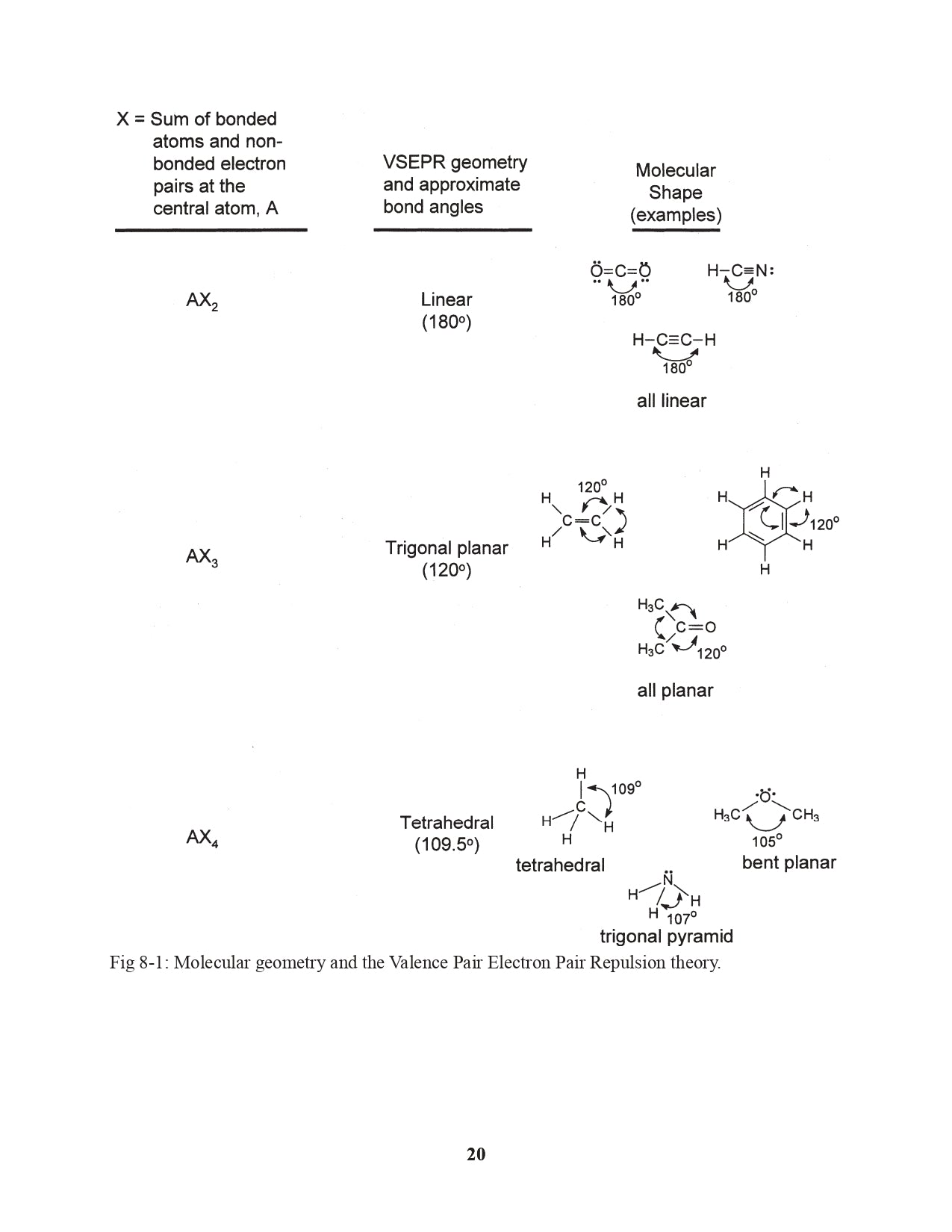
Chapter 8. Molecular Shapes: Valence Shell Electron Pair Repulsion Theory
Chapter 9. Atomic Orbitals
Chapter 10. Orbital Hybridization
Chapter 11. The Functional Groups
Chapter 12. Physical Properties and Molecular Structure
Chapter 13. Lewis Acids and Bases
Chapter 14. Isomers and Stereochemistry
Chapter 15. Introduction to Organic Reactions
Chapter 16. Organic Reaction Mechanisms: A General Overview
Chapter 17. Steric Hindrance
PART 2. THE FUNCTIONAL GROUPS: PROPERTIES AND REACTIVITY
Chapter 18. The Alkane Hydrocarbons
Chapter 19. Alkyl Halides: Substitution and Elimination Reactions
Chapter 20. The Unsaturated Hydrocarbons: Alkenes and Alkynes
Chapter 21. Free-Radical Reactions
Chapter 22. Alcohols and Ethers
Chapter 23. Addition and Substitution Reactions of Aldehydes and Ketones
Chapter 24. Oxidation and Reduction Reactions of Carbonyl Compounds
Chapter 25. Carboxylic Acids and Derivatives
Chapter 26. Reactions of the Carbonyl Group
Chapter 27. Resonance Structures and Electron Delocalization
Chapter 28. Aromatic Compounds and Aromaticity
Chapter 29. Electrophilic Aromatic Substitutions
Chapter 30. Reactions of Enolate Carbanions
Chapter 31. The Amines
Chapter 32. Overview of Reaction Mechanisms: The Production and Fate of Reactive Intermediates
PART 3. THE SPECTROSCOPIC METHODS OF ANALYSIS
Chapter 33. Introduction
Chapter 34. U-V Visible Spectroscopy
Chapter 35. Infra-Red Spectroscopy
Chapter 36. Proton NMR Spectroscopy
Chapter 37. Carbon-13 NMR
Chapter 38. Mass Spectrometry.